SOLVED: What is the balanced equation and the net ionic equation. NaH2PO4 + Na2HPO4 + H2O + MgCl2 ⇒ NaH2PO4 + Na2HPO4 + H2O + NaCl ⇒ ( we did an experiment
SODIUM DIHYDROGEN PHOSPHATE STARTING FROM SODIUM CHLORIDE AND ORTHOPHOSPHORIC ACID VIA CATION RESIN EXCHANGE Doan Pham Minh, A

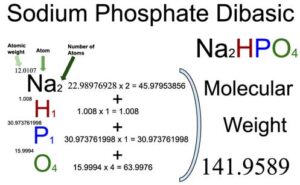
1 Bán Hóa chất di-Sodium hydrogen phosphate dihydrate, - Na2HPO4.2H2O - SO0339 - Scharlau giá rẻ ở hcm
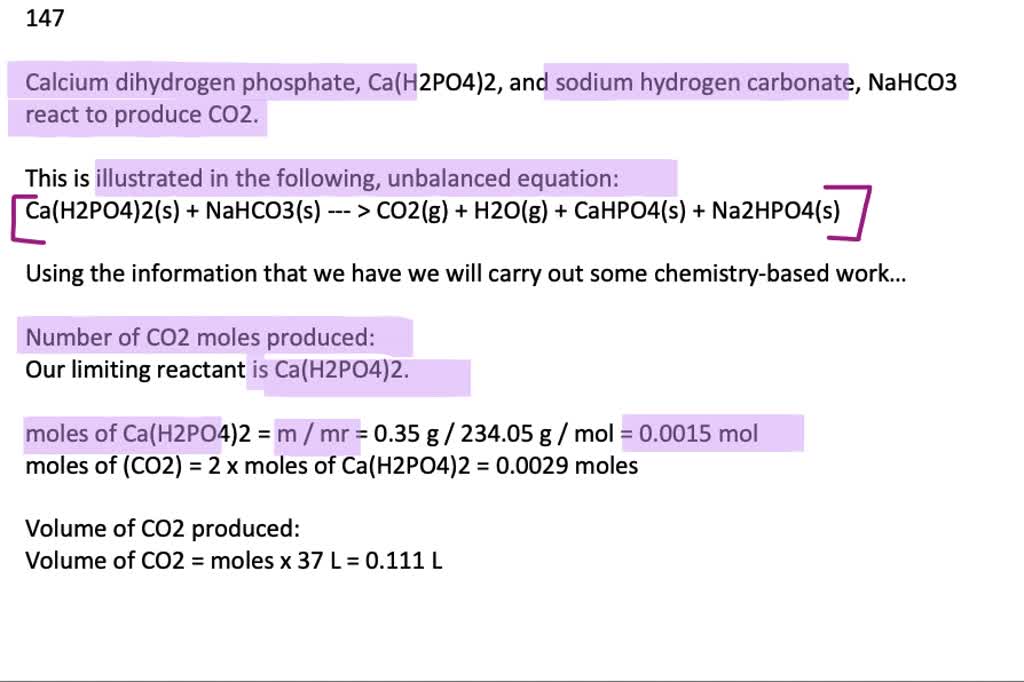
SOLVED: Calcium dihydrogen phosphate, Ca(H2PO4)2, and sodium hydrogen carbonate, NaHCO3, are ingredients of baking powder that react with each other to produce CO2, which causes dough or batter to rise: Ca(H2PO4)2(s) +

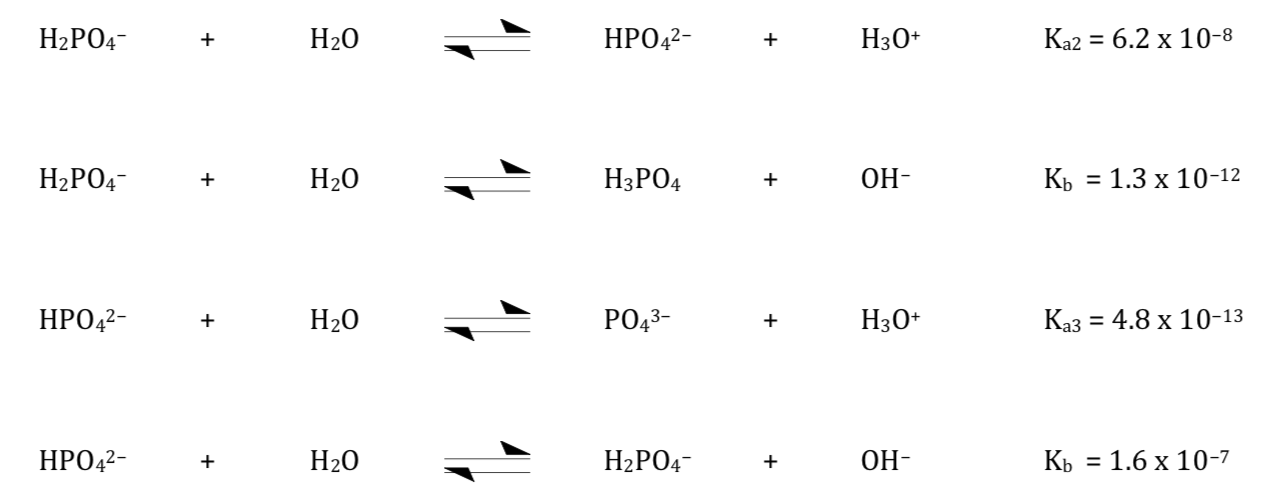
Equilibrium constants is given (in atm) for the following reaction 0^∘C : Na2HPO4. 12H2O(s) Na2HPO4. 7H2O(s) + 5H2O(g) ; Kp = 2.43 × 10^-13 The vapour pressure of water at 0^∘C is

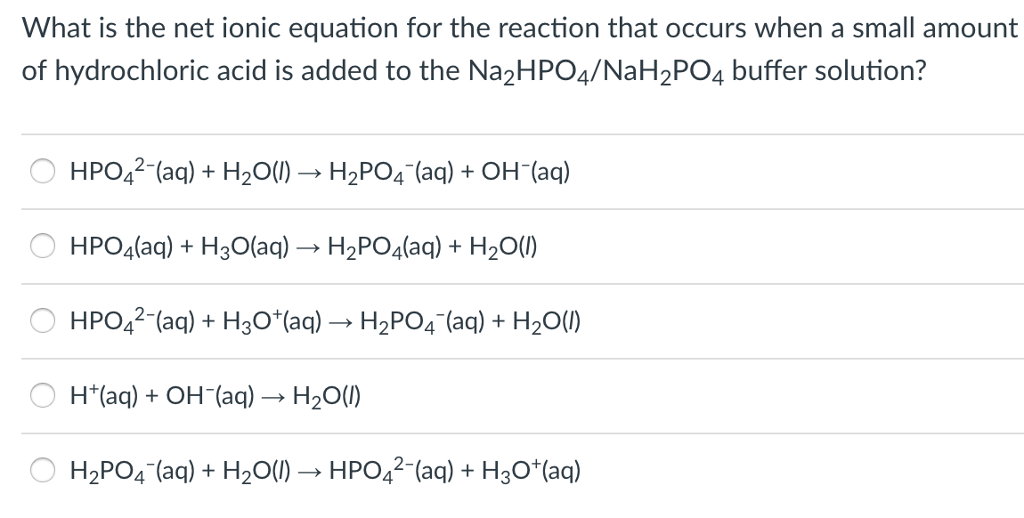


2MoO4%20+%20HNO3%20=%20(NH4)3(PMo12O40)%20+%20NH4NO3%20+%20NaNO3%20+%20H2O.svg)