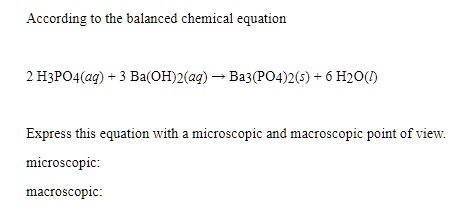
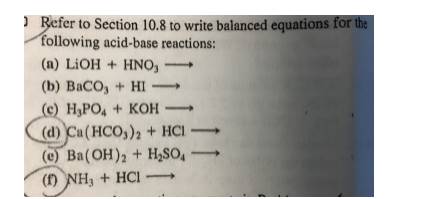
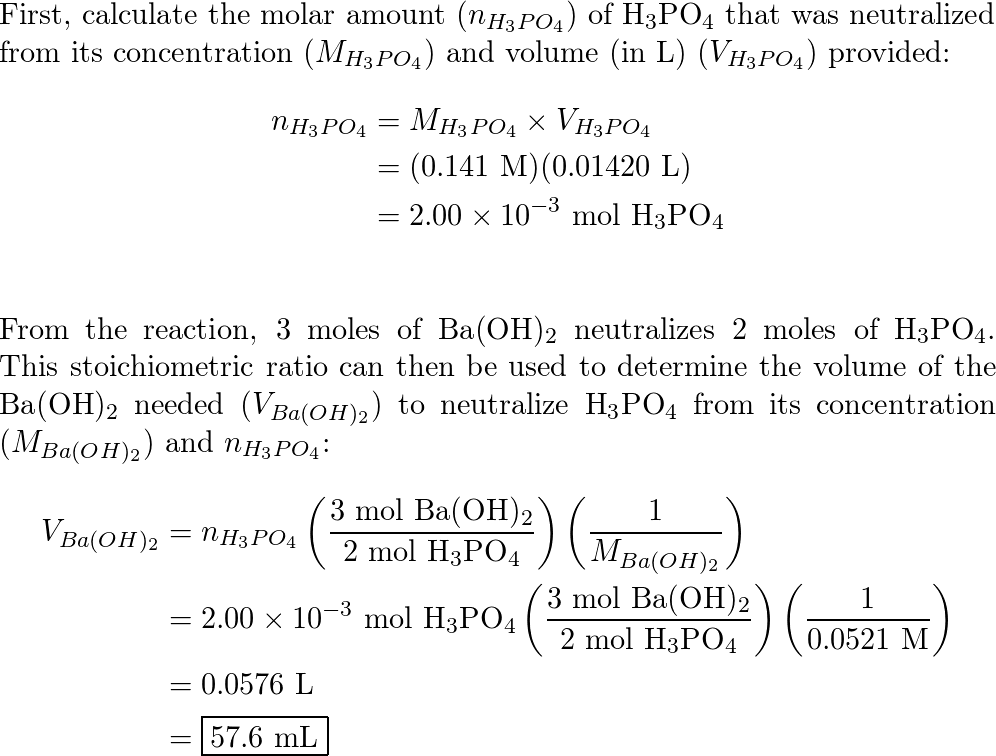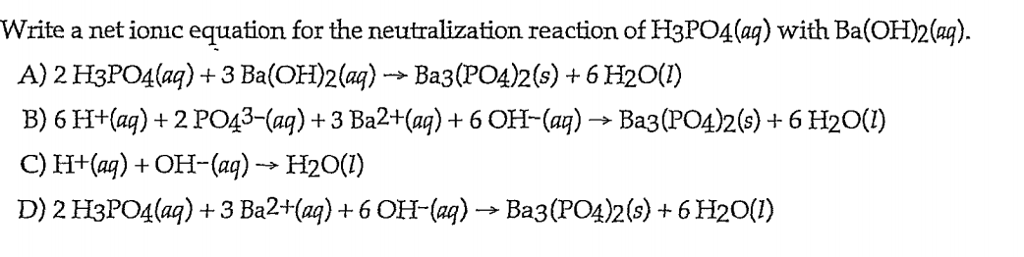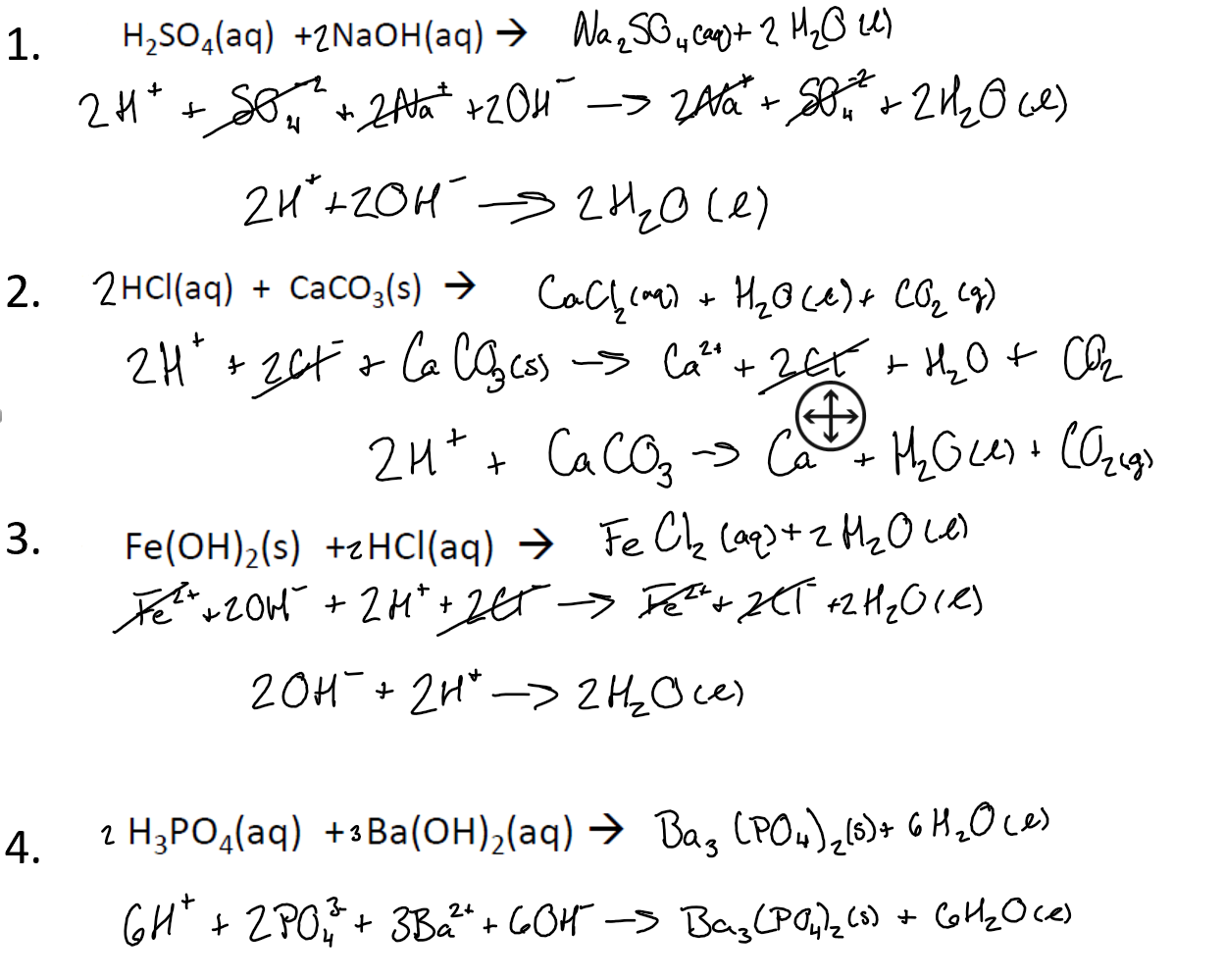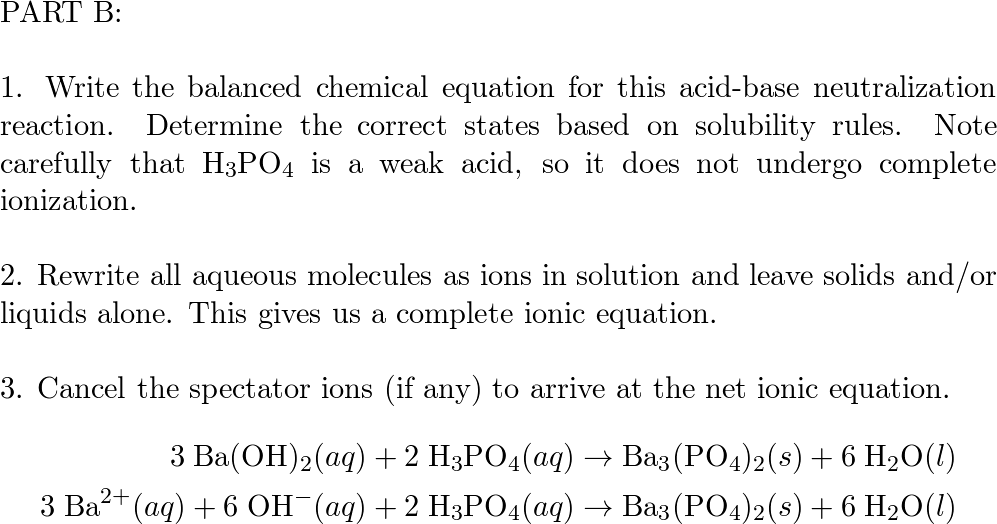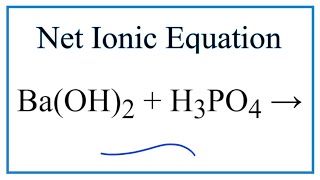SOLVED: Barium hydroxide and phosphoric acid react as follows: 3 Ba(OH)2(s) + 2 H3PO4(aq) –> Ba3(PO4)2(aq) + 6 H2O(l) If 39.5 g of Ba(OH)2 are allowed to react with 51.0 g of
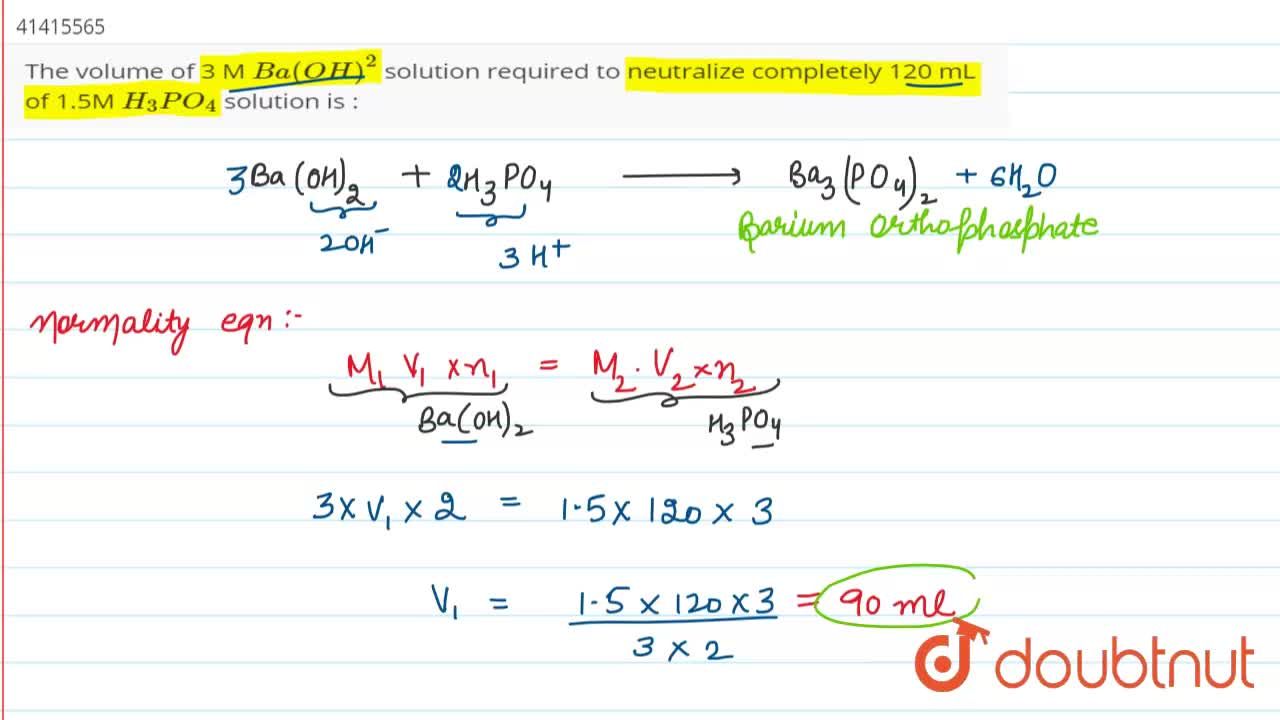
The volume of 3 M Ba(OH)^(2) solution required to neutralize completely 120 mL of 1.5M H(3)PO(4) solution is :
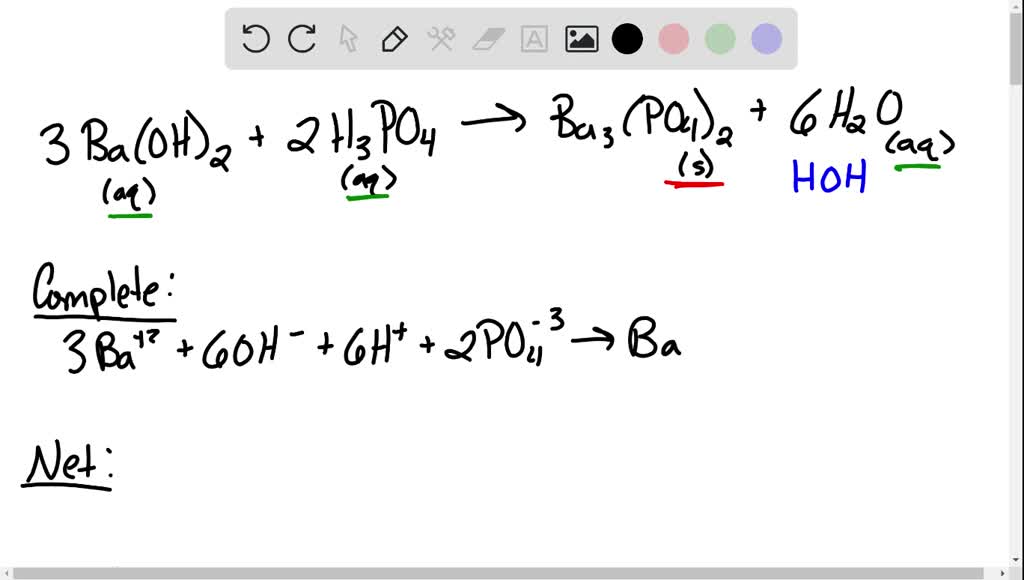
SOLVED: What would be the total ionic equation for 3Ba(OH)2 (aq) + 2H3PO4 (aq) → Ba3(PO4)2 (s) + 6H2O (aq)? Identify the spectator ions of this reaction.

If 15.0 mL of 12.0 M H3PO4 reacts with 100.0 mL of 3.50 M of Ba(OH)2 , which substances is the limiting - Brainly.com
✓ Solved: What volume of 0.0521 M Ba(OH) 2 is required to neutralize exactly 14.20 mL of 0.141 M H 3...