The reactant which is entirely consumed in the reaction is known as limiting reagent. In the reaction 2A + 4B → 3C + 4D , when 5 moles of A react with

Question Video: Calculating the Moles of a Reactant Consumed in a Reaction Given the Moles of a Second Reactant | Nagwa

5 mole of an ideal gas expand isothermally and irreversibly from a pressure of 10 atm to 1 atm against a constant external pressure of 1 atm. w irr at 300K is :

Show by means of calculations that 5 moles of `CO_(2)` and `5` moles of `H_(2)O` do not have the - YouTube

The temperature of 5 mol of gas which was held at constant volume was change from 100^(@)C to 120^(@)C. The change in internal energy was found to ve 80 J. The total
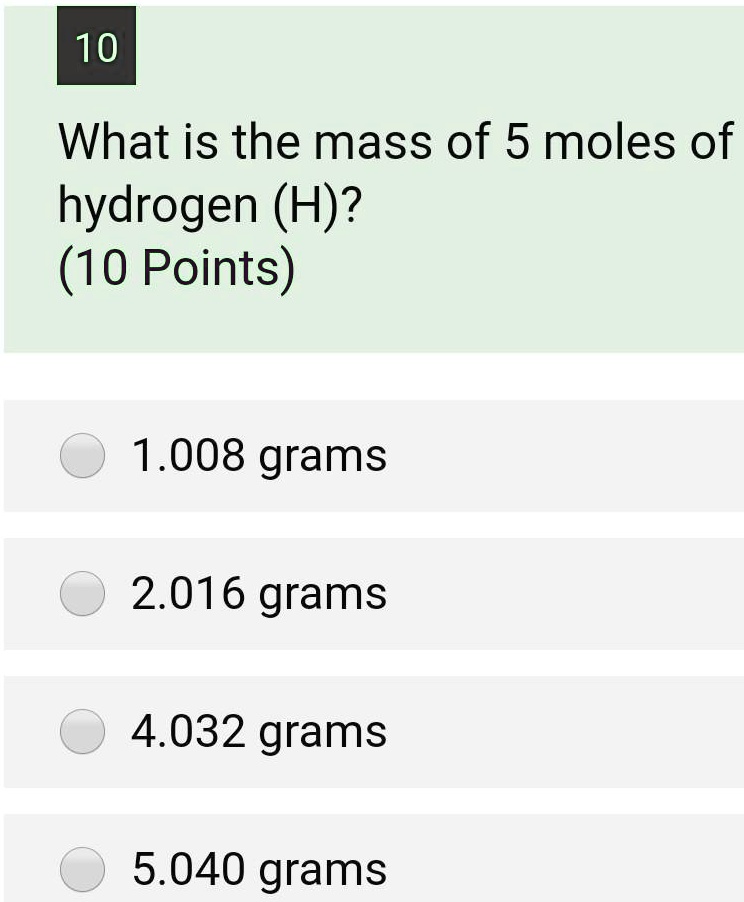
SOLVED: 10 What is the mass of 5 moles of hydrogen (H)? (10 Points) 1.008 grams 2.016 grams 4.032 grams 5.040 grams




![Borax Pentahydrate 5 Mol [Na2B4O7.5H2O] [CAS_11130-12-4] Technical Sta – Wintersun Borax Pentahydrate 5 Mol [Na2B4O7.5H2O] [CAS_11130-12-4] Technical Sta – Wintersun](https://cdn.shopify.com/s/files/1/0724/7981/products/02-010-4_1024x1024.jpg?v=1532727979)